About
Viage Therapeutics, a neuroscience company based on a platform of novel neurotherapeutics, was founded in 2018. The company is based on the ground-breaking research of Dr. Kousaku Ohinata from Kyoto University.
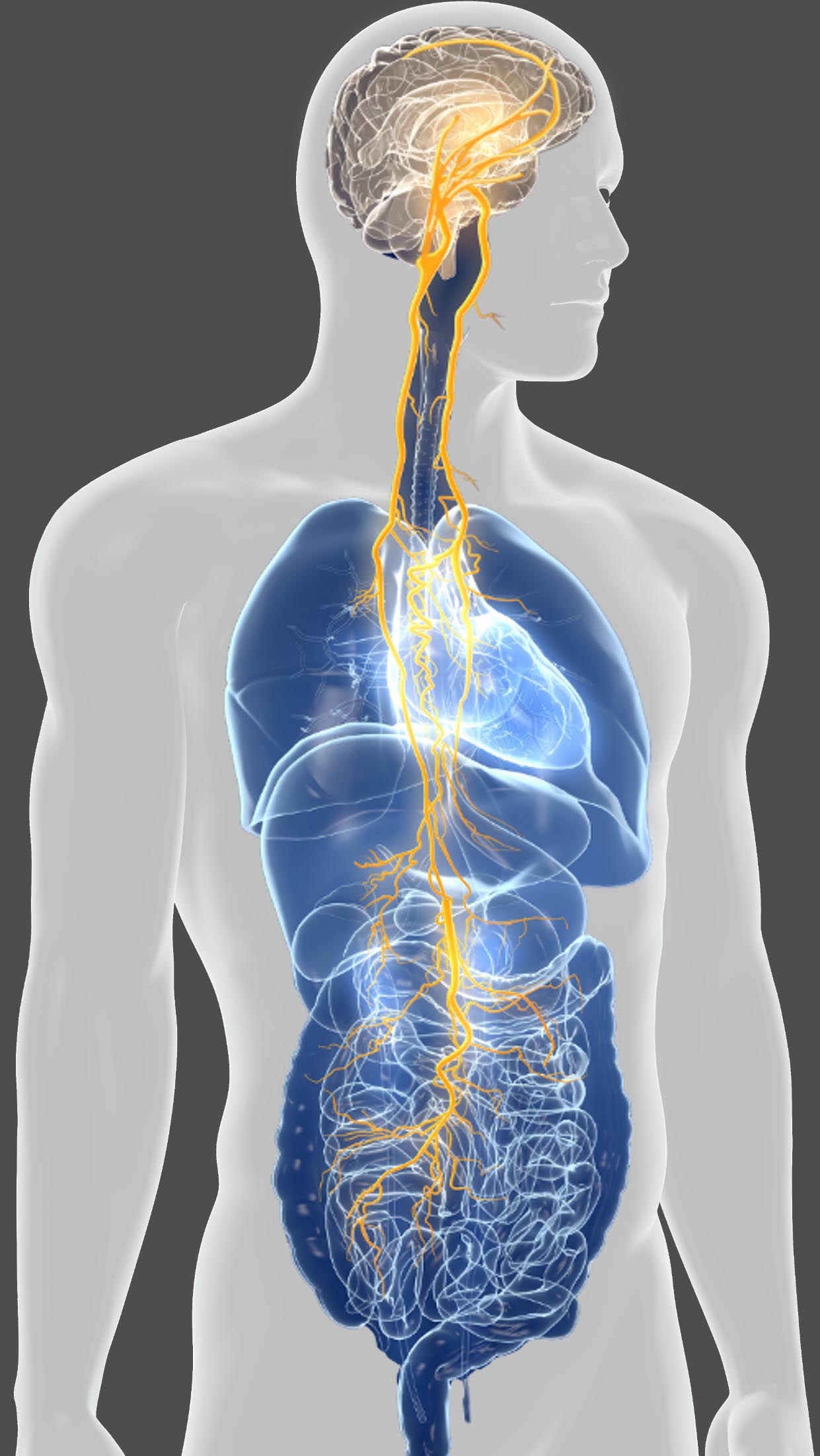
DGX-001, Viage’s clinical drug candidate, represents a first-in-class oral drug candidate targeting the gut-brain axis. The proposed mechanism of action of DGX-001 is to modulate the vagus nerve through specific receptor interactions on enteroendocrine cells in the gut, resulting in a regulation of brain cell activity.
DGX-001 is in clinical development and was well-tolerated in a healthy volunteer Phase 1 study, consistent with its positive safety profile in preclinical GLP studies. In the clinical study, DGX-001 increased specific brain activity (EEG measures) and a post hoc analysis indicated improvements in cognition based on Cogstate tests. Furthermore, human PK measurements have so far been undetectable systemically, confirming the preclinical finding the drug candidate is gut-restricted and gut-acting, which may further minimize off-target side effects as majority of PD patients suffer from comorbidities and polypharmacy.
Viage plans to initiate the Phase 2 program of DGX-001, which initially aim to achieve cognitive improvement across different diseases, representing a transdiagnostic study design, which may ultimately enable indications beyond neurology and psychiatry.
Viage’s pipeline is comprised of both novel neuropeptides and 2nd generation DGX-001-derived peptides and small molecules. It includes SAR-optimized drug candidates with potential in cognitive impairment, depression, anxiety, and inflammatory disease. The drug candidates are gut-acting and gut-restricted, which suggests an advantageous safety profile, particularly compared to most approved psychiatric drugs which suffer from numerous side effects.
The Company is headquartered in the San Francisco Bay Area.
Leadership Team
Titus Plattel, MSc., MBA
30+ years across biotech & life science sectors. Strong operational expertise in start-up companies. Previous leadership positions at Biogen, Amgen, Bavarian Nordic and emerging biotech companies.
Brian Rogers, Ph.D., DABT
30+ years of toxicology experience supporting product development from early stage to NDA filing. Scientist / Toxicologist at Genentech and Toxicologist / Study Director at Chevron. Extensive experience with FDA and authored 100+ IND’s, NDA’s and BLA’s non-clinical sections.
Neil Schwartz
10+ years of experience in R&D drug development in neurology, immunology and inflammation. R&D leadership positions at Epiodyne and Transquis. Extensive research expertise in drug discovery, target identification and SAR development in small molecules and biologics.
Sujata Sankar, Ph.D., MBA
30+ years drug development experience. Senior Operations Executive with experience in program management, clinical operations and general management. Extensive experience in authoring IND’s and full operational responsibility for clinical study development and execution.
Steven King, MSc.
20+ years as public company CEO and board member. Led formation of biologics CDMO Avid Bioservices from inception through $55M+ in revenue for products commercialized in over 70 countries. Led R&D efforts to advance programs from R&D through Phase 3 clinical trials.
Advisors
Kousaku Ohinata, Ph.D.
Associate Professor at Kyoto University with 20+ year research focus on physiologically active substances from food sources. 80+ peer reviewed publications; 40+ patents.
Larry Ereshefsky, Pharm.D., BCCP, FCCP
CSO at Apex Innovative Sciences, proven record leading Phase 1/2a studies CNS indications. Pioneered neurocircuitry / biomarker-based studies incorporating qEEG, ERP, fMRI, etc. 45+ years experience, PI for 100+ studies, 2 terms on the FDA Psychopharmacological Drugs Advisory Committee, several terms on the USP-Panel for Psychiatry.
Harald Murck, MD, Ph.D.
20 years of drug development experience from Stage I to Stage IV with focus on translational and biomarker research. Previously at Sage, Acorda, Axovant, Novartis and others. Adjunct Professor for Psychiatry at the Philipps-University Marburg, Germany, with 85+ peer reviewed articles.
News
Viage Therapeutics announces data from a Phase 1 study with DGX-001, a first-in-class oral neurotherapeutic targeting cognitive impairment in patients with Alzheimer's Disease and Parkinson's Disease
Viage Therapeutics (Viage), a neuroscience company based on a platform of novel neurotherapeutics, today announced positive findings from its Phase 1 study of DGX-001, a first-in class oral therapeutic targeting the AVPR1A receptor in the gut. DGX-001 demonstrated both a favorable safety profile and proof of concept data relating to its mechanism of action (MoA), which modulates the vagus nerve via specific receptor interactions on enteroendocrine cells in the gut, with no systemic exposure of the drug and no need to penetrate the blood brain barrier.
Digestome Therapeutics Announces First Patient Enrolled in Phase 1 Clinical Trial of DGX‑001
Digestome Therapeutics, a biotech company developing first-in-class therapeutics that stimulate the central nervous system (CNS) via the gut-brain-axis, announced that the first patient has been enrolled in a Phase 1 clinical trial investigating DGX-001. The trial will feature safety as the primary endpoint and will also investigate a variety of biomarkers to assess the biological and clinical response to DGX-001.
Digestome Therapeutics Announces Exclusive Out-license of DGX-001 for Greater China with Zhongze Therapeutics
Digestome Therapeutics, a biotech company developing first-in-class therapeutics that stimulate the central nervous system (CNS) via the gut-brain-axis, announced today the completion of an exclusive out-licensing agreement of DGX-001 in the greater China territory with Zhongze Therapeutics.
Digestome Therapeutics Completes $10 Million Seed Financing
Digestome Therapeutics, a biotech company developing first-in-class therapeutics that stimulate mind via the gut-brain-axis, announced today the completion of a $10M Seed financing to advance its lead program into clinical trials starting early 2022.
Hey, There's a Second Brain in Your Gut
New research reveals that the “second brain” in your gut has at least a dozen kinds of neurons, unlocking new paths to further experiments and findings.
